CD-241, FRED M. LOCKE (and his Pyrex Connection)
Reprinted from "Crown Jewels of the Wire", December 1989, page 6
I first learned about this insulator and Fred Locke's possible connection
with Corning's boro-silicate Pyrex glass several years ago from Lew Hohn. Two of
the three known specimens were at the Houston National in 1988. The specimen
shown in the photograph below is a light sage-aqua and the second specimen is
identical. The third specimen (pictured on the fourth page) was in a display at
the Houston National. It is a light lemon-clear glass. All three insulators
have an extended inner skirt and the upside-down embossing as shown below:
Front: PATD BY F.M.LOCKE
VICTOR
N.Y.
Back: MARCH 31 1914 FEB 2 1915
OCT. 12, 1915

While there has been considerable speculation as to the manufacturer, due to
natural curiosity and the unusual glass colors, it should be understood that
such speculation is futile. As you will see after this discussion, the glass
cannot be like other standard production.
The patent dates on CD-241 were granted to Fred Locke for boro-silicate glass
(more on these patents later). This type of glass is now commonly referred to by
the trade name "Pyrex". The Pyrex trademark was registered to Corning
Glass Works on May 5, 1925. The application for the Pyrex trademark stated that
the Pyrex name had been used since June 16, 1923 for electrical insulators and
electrical insulating compounds. However, I am fairly certain that CD-241 was
not made by Corning.
The research into Locke's possible connection with Corning's boro-silicate
Pyrex glass is quite complex. This article was written in an effort to document
the information, and I hope that it will also be interesting. We may never know
with certainty if Fred Locke's patents for boro-silicate glass were indeed used
by Corning and initiated their use of Pyrex glass for insulators.
It is unknown why Fred Locke left the Locke Insulator Corp. in late 1903. One
speculation is that his company became too large for him to manage and still
have time to experiment with new ideas. While his obituary said that he retired
in 1904 (at the age of 43), he did become associated with the Lima Insulator
Corp. when that company was formed in 1904. His name appeared in various trade
journal articles in direct association with the Lima Insulator Corp and he may
have acted as a consultant for them. That association was soon ended when the
Lima plant burned in 1908.
However, Fred Locke remained very active in experimentation with various
insulator designs and material compositions. He was granted 56 patents from 1889
to 1933, 32 of which applications were filed after he left the Locke Insulator
Corp. I recently discovered seven of his last patents after a lengthy patent
search as the result of a tip from Jeff McCurty. [Jeff's interest in Corning and
CD-241 prompted a chain of events leading to the writing of this article.]
Fred Locke was undoubtedly well compensated for his share of the company when
he left. His obituary stated that he maintained a large laboratory at his home
in Victor, N.Y. There he surely spent countless hours experimenting, initially
with new insulator designs. Soon he was experimenting with adding boron to clay
mixtures to increase the strength of porcelain insulators. After promising
results, he then began experimented to see if boron would also improve the
properties of glass.
After initial successes, from about 1909 until 1917, Fred was engrossed with
finding the right formula to strengthen glass by adding boron to glass batches
and he attempted to cover all possible bases with a number of patents for boro-silicate glass. Then, until his death in 1930, he apparently
was searching for a way to make colorless boro-silicate glass.
Fred Locke was granted 12 patents using boron compounds in the manufacture of
porcelain and glass insulators to improve the electrical and temperature
resistance properties.
|
Granted |
Filing Date
|
|
|
1,091,678
|
3-31-1914
|
5-19-1909
|
Boron in porcelain, glass
|
|
1,091,679
|
3-31-1914
|
3-9-1909
|
Boron in porcelain, glass
|
|
1,120,951
|
12-15-1914
|
6-2-1914
|
Boron in porcelain
|
|
1,127,042
|
2-
2-1915
|
3-9-1909
|
Boron in porcelain, glass
|
|
1,127,044
|
2- 2-1915
|
3-9-1914
|
Boron in porcelain, glass
|
|
1,156,163
|
10-12-1915
|
3-9-1909
|
Boron in porcelain, glass
|
|
1,225,147
|
5- 8-1917
|
9-4-1914
|
Boron in glass
|
|
1,226,088
|
5-15-1917
|
6-16-1914
|
Boron in porcelain, glass
|
|
1,233,486
|
7- 7-1917
|
8-28-1916
|
Specific glass formulas using boron
|
|
1,431,166
|
10-10-1922
|
9-16-1916
|
renewed 1-21-1922, Boron in glass
|
|
1,510,521
|
10-7-1924
|
9-16-1916 |
renewed 4-3-1922, Boron in glass |
|
1,626,042
|
4-26-1927
|
8-28-1916 |
renewed 10-29-1926, Boron in glass |
Most of these patents deal with broad claims of using boron in both porcelain
and glass insulators. All but a couple of the patents are indistinguishable as
the text is nearly word-for-word with only minor changes. Patent #l,225,147
described testing 10" suspension disks made from the new glass. Patent #l,233,486 gives specific glass formulas for actual insulators that he used for
experiments. It also claimed making clear (colorless) glass by adding the
nitrate of either potassium or sodium. The making of cheap, clear glass may be
the key to Fred's involvement with Corning.
My guess is that Fred initially experimented with improving the electrical
properties of porcelain insulators. Then, later, after seeing the success he had
with porcelain, decided to see if the electrical properties of glass could also
be improved by adding boron. That much is fairly evident when reading the
initial patents where the emphasis is on porcelain insulators. Early trade
journal articles (shown later in this article) about Locke's new insulator
material refer to "transparent porcelain" and "boro-porcelain".
Fred still had in mind making porcelain even though the products shown in the
trade journals were obviously glass. He evidently had sufficient equipment in
his laboratory to make insulators for testing purposes -- suspension disks,
bushings and clear glass tubes. It was even stated in one article that the
"...material is being placed on the market by Fred.......". That does not necessarily mean that he
was selling insulators, but could merely indicate that he was trying to market
the patents.
The three known insulator specimens of CD-241 were probably made in his
private laboratory! Knowing Locke's great desire to show all the current patent
dates on an insulator, the three specimens were probably made between October,
1915 and May, 1917. Locke would surely have marked them with the three 1917
patent dates if they had been granted before the insulators were manufactured.
The three patent dates shown on the insulator actually represent five patents.

It is known, through present day Locke relatives (via Lew Hohn's contact with
them), that Fred received royalties from Corning Glass Works for "many
years" for his glass patents. Corning was also working on glass formulas
with boron compounds as early as 1914. Their second patent was clearly
interested only in various laboratory dishes and glassware. Since Corning was
also working on boro-silicate glass formulas, they may have purchased the rights
to Fred's boro-silicate glass patents to protect their use of boro-silicate
glass in Pyrex products. We may never know for sure if Locke should be credited
with patenting Pyrex glass since his glass patents were before Corning's glass
patents, but his obituary in 1930 stated one of his greatest accomplishments
was "...heat enduring glass."
Jeff's contacts at Corning said that Pyrex glass was the result of their 1919
patent. Commercialization of Pyrex may not have been practical until their 1923
patent which coincides with the development of the Pyrex trademark. The Corning patents are:
|
Granted |
Filing Date |
|
|
1,192,474
|
7-25-1916
|
9-17-1914
|
Boron in glass
|
|
l,304,623
|
5-27-1919
|
6-24-1915
|
Boron in glass (several specific formulas for
laboratory dishes)
|
|
1,449,793
|
3-27-1923
|
11-29-1920
|
Adding neodymium oxide to boro-silicate
glass to overcome yellow tint from iron
|
Corning wanted a clear boro-silicate glass but they could not find a way to
make it cheaply. Iron, naturally present in sand, produces a green (aqua) tint
to ordinary glass and a yellow tint to boro-silicate glass. While either
manganese, nickel or selenium were used to decolorize (make clear) ordinary
glass (with excess quantities producing pink or SCA glass), these elements would
only intensify the yellow tint of boro-silicate glass making it amber. Corning's
1923 patent used neodymium oxide to overcome the yellow tint and produced a
clear bore-silicate glass. This compound must have been expensive in the
quantities needed for production. The patent called for a commercially available
source which only partially contained neodymium.
Corning's boro-silicate patents showed only an interest in laboratory dishes.
Jeff McCurty's research into Corning's history indicates that they had ideas to
use glass for insulators as early as 1913, started working with suspension
insulators in the early 1920's and had no interest in pin types until around
1925. The first sample pin types were not made until 1926. Could Corning have
become more interested in making insulators after being approached by Locke in
1926 with the solution to producing a cheap, clear boro-silicate glass? Locke's
last two patents may be the key to Corning entering into the insulator business.
Note that the first patent was filed in early 1926 -- the same year that Corning
produced their first pin types samples.
One of his five sons, Fred J. Locke, joined him as a co-worker in his
laboratory, probably around 1920, and was named co-patentee on four patents.
Fred N. Locke and Fred J. Locke had their crowning achievement with their last
two patents:
|
Granted |
Filing Date |
|
1,886,280
|
11- 1-1932
|
4-13-1926
|
refiled 1-17-1927, for a
method to produce colorless boro-silicate glass that will transmit ultraviolet
light by adding fluorine to a batch of glass in a carbon pot.
|
1,923,221
|
8-22-1933
|
3-23-1931
|
for a method to produce a colorless boro-silicate or other
glass that will transmit ultraviolet light by adding fluorine and antimony to a
batch of glass in a carbon pot.
|
Both patents were assigned to Corning Glass Works. These are the only Locke
patents known to have been assigned to Corning. Others may have been purchased
by Corning. A very interesting note is that both patents were granted after
father and son had died. Fred's wife, Mercie P. Locke, was listed on both
patents as the executrix and administratrix. Fred M. Locke's obituary stated
that he died on April 16, 1930 at his home in Victor, N. Y. just 8 days short of
being 69 years old. The recent death of his son and co-worker, Fred J., on March
7, 1930 "...undoubtedly shortened the life of the inventor who vas the
originator of many devices and materials besides two which are of paramount importance, the Locke insulator and heat enduring glass." The "heat
enduring glass" evidently referred to boro-silicate glass. Note that the
last patent granted to Locke was filed nearly a year after is death and was a
continuation of a patent application they had filed July 18, 1929. Corning may
have become interested in Locke's last two patents as the result of the
economical method to produce colorless boro-silicate glass. However, Corning's
interest in these two patents may have been the superior optical quality of the
glass -- better transmission of ultra-violet light -- and the ultimate use in
the mirror for the 200 inch Mt. Palomar telescope manufactured by Corning in
1934.
It appears from Corning's vast patent record, during the years 1915 to 1930,
that their major interests were lens for automobile headlights, opaque
flesh-colored glass, glass that would absorb ultraviolet light, colored glass,
vases and many different types of serving and cooking dishes, bowls and cups. It
was not until after Locke's 1926 patent application for producing economical
colorless glass that Corning entered into the pin-type insulator manufacture. Is
there a connection here?? Corning's "Pyrex" tradename has been used to cover
the manufacture of many boro-silicate glass formulas including insulators,
laboratory ware and ovenware.
The variation in color of the three CD-241 insulators (sage-aqua and light
lemon-clear) is undoubtedly due to different glass formulas with which Fred was
experimenting and the persistent impurity of iron oxide in the sand. The glass
would naturally appear like no other standard production, and could not be
compared with any manufacturer's production to enable one to guess the name of
the manufacturer. These insulators were probably made for experimentation by
Locke in his laboratory.
To further show Fred Locke's interests with his early boron formulations for
glass and porcelain, copies of the following trade journal articles are included:
|
Electrical World,
|
6-24-1909
|
("Transparent Porcelain") |
|
Electrical World,
|
4- 3-1915
|
("Boro-porcelain") |
|
Electrical World,
|
5-29-1915
|
("Boro-silicon") |
|
Electrical World,
|
1-8-1916
|
("Boro-porcelain") |
I want to thank Jeff McCurty for his assistance in this project. He supplied
the "key" tip (as well as other information) from his source at
Corning, namely, that Locke had patented Corning's Pyrex "Flameware"
glass in 1925. Without his tip on this unknown Locke patent, many of Fred
Locke's last patents would not have been discovered by me, and the story as we
know it todate would have yet to be written. Corning bought the rights to this
glass patent and later worked on the tempering process. Pyrex Flameware was
first used for cooking dishes in 1936 and later in the Apollo and Gemini space
programs and the X-15 rocket plane. This is another "heat enduring"
glass referred to as alumino-silicate glass. It is high in alumina, low in
silica (sand) and contains no boron compounds. The patent is:
|
Granted |
Filing Date |
|
|
1,529,259
|
3-10-1925
|
12- 9-1922
|
granted to Fred M. and
Fred J. Locke
|
The following is from Electrical World dated 4-3-1915.
|
Insulating Composition Material
An insulating material containing boron,
which is called "boro-porcelain," is being made by Fred M. Locke,
Victor, N. Y. With this material a low dielectric constant and high dielectric
and mechanical strength are obtained and the coefficient of expansion is almost
as

low, it is declared, as fused silica. According to the manufacturer, the
voltage required to arc over the surface of an insulator made of this, material
is 20 per cent to 30 per cent more than for one of high-grade porcelain, and the
dielectric strength is 50 per cent greater than that of porcelain. It is
especially adapted for use with high-voltage transmission lines.
|
The following is from Electrical World dated 6-24-1909.
|
New Type of Locke High-Tension Insulator
Fred. M. Locke, Victor, N. Y., announces that he has succeeded in making a
new material for insulators which he calls "Transparent Porcelain,"
and which possesses a number of remarkable qualities. The base is aluminum silicate. and the
material
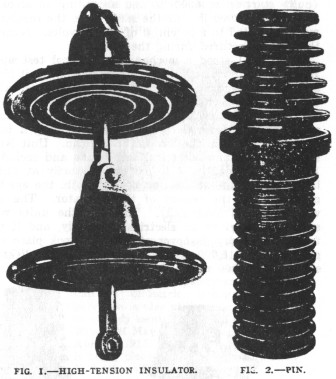
can be melted and cast in the same manner as glass. Two of its most
remarkable features are the temperature changes it will withstand, and its high
specific inductive capacity. These, together with great mechanical strength
and high resistivity, ideally fit the material for all classes of insulation.
The insulation shown in the accompanying illustration has been submitted to the
following tests: Dry arcing at over 105,000 volts per section ; wet arcing at
over 55,000 volts per section; breaking test, 10,700 lb.; sudden change of
temperature from 40 deg. to 200 deg. Fahr. without cracking. Its specific
inductive capacity was found to be 10 as compared with air as unity, the
corresponding figures for glass and porcelain being 2 and 4 respectively.
The
bushing shown in the illustration was tested to 100,000 volts, its flash-over
point, with scarcely any evidences of static effect. It is stated that a large
number of the new insulators have been tested to 100,000 volts for several hours
without any failures.
|
The following is from Electrical World dated 5-29-1915.
|
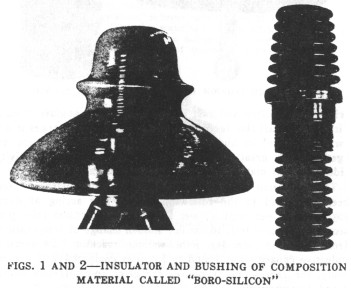
Insulator Tests
Insulators of a special composition material called "boro-silicon,"
which was described in the ELECTRICAL WORLD of April 3, have recently been put
under tests, and the results obtained are reported as follows:
Four 11-in.
suspension units were tested, and each unit was subjected to an increasing
mechanical strain (pull) starting at 6000 lb. and ascending in steps of 1000 lb.
and 2000 lb. At the same time, the insulators were subjected to a potential of
60,000 volts. None of the units punctured during the tests.
Unit No. 1 stood a
mechanical electrical test up to 12,000 lb. without any sign of break-down. Unit
No.2 stood a mechanical test up to 11,000 lb. Just after 12,000 lb. bad been
applied the unit pulled apart, shearing the material evenly in a cleavage plane
at an angle about 60 deg. from the vertical and reaching a point about 0.5 in.
from the bottom of the cap. Unit No.3 stood a mechanical-electrical test up to
and including 12,000 lb. Unit No. 4 tested satisfactorily at 11,000 lb., but
upon the application of 12,000 lb. the eye-bolt pulled out of the cement of the
insulator. The unit was still good electrically. Three of the units were
subsequently given an electrical test only, and it was found that a dry
flashover of one disk took place at a pressure of 115,000 volts, and of two
glass disks in series at 198,000 volts. All the insulators were tested
under oil without puncture. The maximum voltage obtainable, owing to leakage
and flashing around the insulator or to the containing vessel, was, however,
only 170,000 volts, and none of the units were punctured at this voltage. The
insulators were made by Fred M. Locke, Victor, N. Y.
|
The following is from Electrical World dated 1-8-1916.
|
Insulating Tubing
In the accompanying illustration are shown several types of
tubing made of an insulating material called "boro-porcelain." The
tubes vary in length

INSULATING TUBING FOR HIGH-VOLTAGE CIRCUITS
from 3 in. to 7 ft. and are designed for use on high-voltage circuits. The
material, the manufacturer claims, has a low electrostatic capacity and high
and uniform dielectric strength. Boro-porcelain is also utilized for
insulators, as already mentioned in the ELECTRICAL WORLD of May 29, 1915, page
1429, and April 3, 1915, page 869. The material is being placed on the market by
Fred M. Locke, Victor, N.Y.
|
|
